Specialists: Selective Plating
26260 Eden Landing Road , Hayward, California 94545
Phone: (510) 781-5588 Fax: (510) 781-5589
Email: tmartin@platron.com
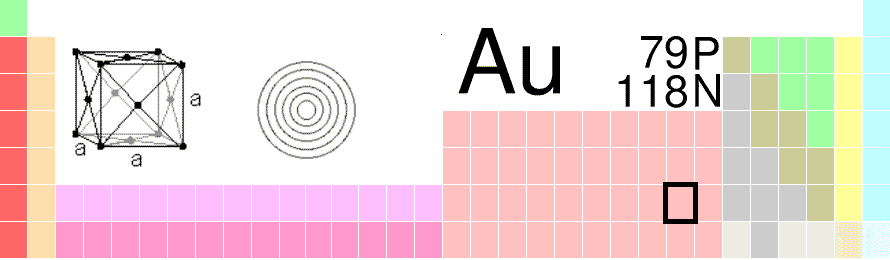
| 79 | platinum ← gold → mercury |
| Ag ↑ Au ↓ Rg |
 |
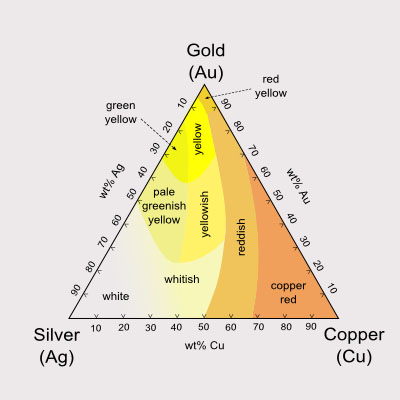
Sliding Color Scale for Gold Alloys.
Gold is a chemical element with the symbol Au with an atomic number of 79. It is a highly sought-after precious metal used in Jewelry and historically for ornamentation. Elemental or pure Gold is dense, soft, shiny and the most malleable and ductile metal; a single gram can be spread out / beaten into a sheet of one square meter. Pure gold has a bright yellow color with a deep shiny luster considered desireable and attractive; it maintains its appearance without oxidizing (rusting) in air or water.
Modern industrial uses include conductivity enhancement in electronics, due to its good resistance to oxidation and corrosion. Chemically, gold is a transition metal. Normally Gold cab be dissolved by aqua regia (a mixture of Nitric and Sulfuric acids) and by alkaline solutions of cyanide but not by single acids. Gold can also dissolve in mercury, but does not react with it. Gold is insoluble in nitric acid, which will dissolve silver and other metals. Gold is a good conductor of heat, electricity and reflects
infra red radiation strongly.
Industrial Uses
• This is typically not an electrodeposited (?) gold but it is the most common use of gold. Gold solder is used for joining the components of gold jewelry by soldering or brazing. If the work is to be of quality, gold solder must match the carat weight of the work, and alloy formulas are manufactured in most industry-standard carat weights to color match yellow and white gold.
• As gold is a good reflector of electromagnetic radiation such as infrared and visible light as well as radio waves, it is used for the protective coatings on many artificial satellites, in infrared protective faceplates in thermal protection suits and astronauts' helmets and in electronic warfare planes.
• Gold is used as the reflective layer on some high end CD’s.
• High end Automobiles use gold for heat insulation.
Electronics
• The concentration of free electrons in gold metal is 5.90×1022 cm-3. Gold is highly conductive to electricity, and has been used for electrical wiring in some high energy applications (silver is even more conductive per volume, but gold has the advantage of corrosion resistance).
• Due to Gold’s good conductivity and general resistance to oxidation and corrosion in harsh environments it enjoys widespread industrial use in the electronics as a thin layer coating electrical connectors of all kinds, thereby ensuring good connection. The use of gold in electronic sliding contacts exposed to a highly humid or corrosive atmosphere, or for contacts with a very high failure cost remains the safe bet and therefore very common.
•Besides sliding electrical contacts, gold is also used in electrical contacts because of its resistance to corrosion, electrical conductivity and its lack of toxicity. Switch contacts are generally subjected to more intense corrosion stress than are sliding contacts.
Silver a chemical element with the symbol Ag and an atomic weight of 47. Silver is a soft, white, lustrous transition metal. It has the highest electrical conductivity of any element and the highest thermal conductivity of any metal. The metal naturally occurs in its pure, free form (native silver) and as an alloy with gold, as well as in various minerals. Most silver is produced as a by-product of refining operations for copper, gold, lead and zinc.
Silver has been known since ancient times and has long been valued as a precious metal, used to make ornaments, jewelry, high-value tableware and utensils (hence the term silverware) and currency coins. Today, silver is used in electrical contacts and conductors, in mirrors and as catalysts in chemical reactions. Its compounds are used in paper based photography and used to make disinfectant compounds. Although the antimicrobial uses of silver have largely been upstaged by the use of antibiotics, further research
into its medical potential is in progress.
Silver is a very ductile and malleable although it is slightly harder than gold. It has a brilliant white metallic luster that can take a high degree of polish. It has the highest electrical conductivity (or lowest electrical resistance) of all metals.
Due to its high demand and limited availability it has a high cost relative to other metals such as copper. Its greater cost and anti-?tarnish ability have prevented it from being more widely, like copper, for its electrical purposes. Among metals, pure silver has the highest thermal conductivity, the whitest color, and the highest optical reflectivity (although aluminum slightly outdoes it in parts of the visible spectrum, and it is a poor reflector of ultraviolet light). Silver also has the lowest contact
resistance of any metal. Silver is stable in pure air and water, but tarnishes (develops a dark oxide surface coating) when it is exposed to air or water containing ozone or hydrogen sulfide.
Old paper based photography used about 24% of the silver consumed in 2001, while 33% was used in jewelry, 40% for industrial uses, and only 3% for coins and medals.(1) However the use of silver in photography does not appear to be declining, despite the switch to digital technology, since in 2007 of the 455.5 million ounces of silver used for industrial applications, over 128 million ounces (28%) were consumed by the photographic sector.(2)
Some electrical applications use silver for its superior conductivity (lowest resistance of all metals), even when tarnished. Printed circuit boards are made using silver paints, and computer keyboards use silver electrical contacts. Some high-end audio hardware is fully silver-wired, which is believed to cause the least loss of quality in the signal. Silver cadmium oxide is used in high voltage contacts because it can withstand voltage arcing.
Nickel is a chemical element, with the chemical symbol Ni with an atomic number of 28. Nickel is a silvery-white lustrous metal with a slight golden tinge. It is one of the four ferromagnetic elements (at room temperature). Nickel is corrosion-resistant, finding many uses in alloys, as a plating, in the manufacture of coins, magnets and common household utensils, as a catalyst for hydrogenation, and in a variety of other applications. Enzymes of certain
life-forms contain nickel as an active center making theemetal essential for them.
Nickel belongs to the transition metals and is hard and moderately ductile. It occurs most often in combination with sulfur and iron. Nickel is similar to the elements chromium, aluminum and titanium. Nickel is a very reactive element, but is slow to react in air at normal temperatures and pressures. Due to its permanence in air and its slow rate of oxidation, it has been used in coins, for plating metals and chemical apparatus.
Industrially nickel is chiefly valuable for the alloys it forms, especially many super alloys, and particularly stainless steel. Nickel is also a naturally magnetostrictive material, meaning that in the presence of a strong magnetic field, the material undergoes a small change in length. In the case of Nickel, this change in length is negative (contraction of the material), which is known as negative magnetostriction.
Nickel is used in many industrial and consumer products, including stainless steel, magnets, coinage, rechargeable batteries, electric guitar strings and special alloys. It is also used for plating and as a green tint in glass. Nickel is pre-eminently an alloy metal, and its chief use is in the nickel steels and nickel cast irons, of which there are many varieties. It is also widely used in many other alloys, such as nickel brasses and bronzes, and alloys with copper, chromium, aluminum, lead, cobalt, silver,
and gold.
The amounts of nickel used for various applications are 60% used for making nickel steels, 14% used in nickel-copper alloys and nickel silver, 9% used to make malleable nickel, nickel clad, Inconel and other super alloys, 6% used in plating, 3% use for nickel cast irons, 3% in heat and electric resistance alloys, such as Nichrome, 2% used for nickel brasses and bronzes with the remaining 3% of the nickel consumption in all other applications combined.
The American nickel ( 5 cent coin) is 75% copper and 25% nickel. The Canadian nickel minted at various periods between 1922-81 was 99.9% nickel, and was magnetic. Various other nations have historically used and still use nickel in their coinage.
1- Butterman, WC & Hilliard, HE (2005) Silver. Open file report 2004-1251. US Geological Survey mineral commodity profiles, Reston, Virginia.
2- http://www.silverinstitute.org/silver_uses.php
3- Los Alamos National Laboratory, Silver, accessed 12 February 2007.